This post is not about aliens, but it is about the night-time marauding behaviour of fierce, many-limbed* predators: candy-striped spiders!
I am very excited to share this new paper that was a collaboration with my partner in life and science, Sean McCann! It’s the shortest paper I’ve ever written, and also my favourite scientific contribution so far. Written in a style that I hope is engaging and accessible, it documents some of the fascinating natural history of candy-striped spiders in North America. It is very short, so I encourage you to go read it in its entirety! Here I will present some back story and behind-the-scenes photos of what went into this study about the foraging behaviour of these intrepid spiders.
It all started with Sean’s penchant for getting up before dawn to take pictures of sleeping insects like these cuckoo leaf-cutter bees:

While we were doing fieldwork as part of my PhD research on black widow spiders on the lands of the Tsawout First Nation on the Pacific Coast, we had a mostly nocturnal lifestyle, but occasionally we would head out to our coastal dune field site early in the morning for some recreational spider and insect observations and photography. While searching for sleeping insects for Sean to shoot, we started to notice that wasps and bees would often be perched on dead vegetation in large aggregations like the one below.

Sometimes multiple species of wasps and bees would be sleeping together on the same dead plant. The stems used for perches were typically isolated from other vegetation, and we started to wonder about the sleeping habits of these insects. Does perching together in large groups reduce the risk of being taken by predators? Do the same bees and wasps come back to sleep together at the same perch night after night?

We decided we would do a little project to try to find some answers. We located a few communal perches within our widow spider study area that we could visit each night during our regular surveys. Then we painted all the insects sleeping on the same perch with a dot of the same colour of paint, so we would recognize them if they returned.

Then over the next few nights, we returned to the perches to count the number of painted insects and new arrivals.

Soon, our ideas about safety in numbers were put to the test. We started to find our bees and wasps being picked off by spiders! In one case (shown below) a single sleeper of a group of 5 was captured and killed by a spider in the night.

In other cases however, we came upon perches whose whole cohort of sleepers had been massacred by a single spider! Now our curiosity turned to the behaviour of the spiders, who we suspected were able to subdue these large, otherwise well-defended (with venomous stings) prey by sneaking up on them while they were sleeping and too cold to defend themselves.

We now wanted to know who these spiders were, and what was known about their predation behaviour. The details are all in the paper, but the short version follows.

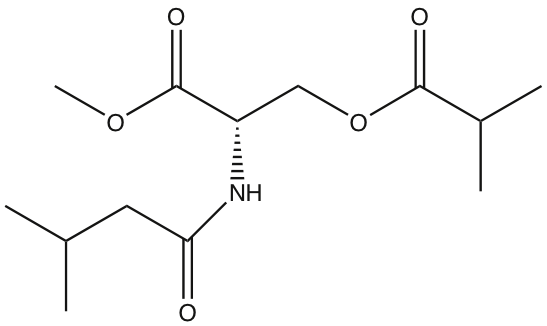
Candy-striped spiders are introduced to North America from Europe, and they can be extremely abundant in many habitats. They come in three colour varieties, and while the genetics of this polymorphism has been worked out, not much attention has been paid to their behaviour. Most of what we know about their predation is based on the observations of William Bristowe, an English naturalist who studied about these spiders almost 100 years ago. He described the following foraging tactics:
Web-based ambush. Like other members of the family Theridiidae, candy-striped spiders build messy tangle webs, often under flowers. When day-flying insects land on the flowers to feed on nectar and make contact with her silk threads, the lurking spider will rush out to wrap the prey with strong, sticky silk.

Web invasion for kleptoparasitism (stealing prey) and araneophagy (spider-eating). Sometimes, candy-striped spiders connect their webs to those of neighbouring spiders to eavesdrop on the vibrations produced by prey insects. At our field site, they would often have lines connecting their webs to those of black widows, and creep onto the web to try to steal prey from their much larger relatives (black widows are in the same family of tangle-web weavers: Theridiidae). They also sometimes eat the spiders themselves!


More recently, candy-stiped spiders have been reported actively foraging without the aid of a capture web. A very cool study found that the spiders eavesdrop on the vibrations produced by sexually signalling leafhoppers and use these to locate these insects as they move around in grasses and shrubs.

Launching sneak-attacks on sleeping insects, however, appears not to have been previously reported. You can find a whole gallery of images and videos of the behaviours we observed here, including some attacks on sleeping bees and wasps that we just happened to witness, and some that we set up.

There’s much more in the paper about the diet of these spiders, and the details of how we studied them, and I hope you’ll go check it out! As for conclusions, this research resulted in a lot more questions than answers. What prompts the decision by these spiders to leave their webs and go marauding? (Presumably it is risky to leave the protection of the tangle web.) Do they all do it, or is this a new or learned behaviour in an environment where sleeping insects are particularly abundant? Do the spiders just search randomly for sleeping prey, or are they able to detect them from a distance (perhaps using chemical cues)? And what is the impact of all this predation on insect communities, particularly in threatened ecosystems like the sand dunes where we worked? These spiders take a lot of pollinators, but they also take all kinds of other bugs—are they impacting pollinator populations, helping to control insect pests in agricultural ecosystems, or both? The list goes on, and we have years of work ahead of us to find out!

Speaking of which, if you’re in North America, YOU can help us with our ongoing work on candy-striped spiders! Please keep your eye out for these spiders, wherever you are, and contribute observations to our iNaturalist project. More information about this participatory research here.
*As far as I can tell, the “queen” alien from the movie has 6 limbs, but apparently James Cameron drew some inspiration from black widows in coming up with her morphology!